Lack of alkaline stability is a pain point that restricts the development of anion-exchange membrane (AEM) water electrolysis for hydrogen production and fuel cell technology. Liming research group of Hubei University proposed N-methyl quinine cationic structure, and the prepared polyaromatic quinuclidinium AEM has high stability and does not degradation at high temperature and high concentration alkaline environment. It is believed that this work will promote the development of new energy technologies such as anion exchange membrane electrolysis of water and fuel cells. This work by professor Ming Li for the corresponding author, published in the Advanced Materials on impact factor (29.1) (the original link: https://onlinelibrary.wiley.com/doi/10.1002/adma.202306675).
At present, the AEM has a small batch of products for sale, if necessary, please contact Wuhan Limo Technology Co., LTD. (Tel/wechat: 13419663615; Taobao store: Wuhan Limo Technology).
Compared with reported AEMs, polyaromatic quinine AEM has high alkaline stability (80℃, 10 M NaOH aqueous solution, no chemical decomposition, no conductivity attenuation) and high dimensional stability (80℃, swelling rate < 10% in pure water, swelling rate < 2% in 10 M NaOH). High OH-conductivity (~ 139.1mS /cm at 80℃) and high mechanical properties (tensile strength: 41.5MPa, elongation at break 50%). The water electrolyzer assembled with nickel alloy electrodes and AEM has A high current density (1.94 A /cm2) at 2.0V. It is believed that this work will pave the way for the development of hydrogen energy technologies such as anion exchange membrane electrolysis of water for hydrogen production and fuel cells.
Due to the absence of hydrogen atoms on the N-methylquinine cationic β2-carbon, the longer distance between α3-carbon and aryl, and the electron-donating role of -CH2CH2 - in the n-methylquinine cationic, the alkaline stability of the n-methylquinine cationic is higher than that of the N, n-dimethylpyridine that has been reported. In fact, density functional theory (DFT) calculations show that because the Gibbs free energy is increased by 9.57 kCal/mol, the OH-anion attacks the cation to get the intermediate complex 2. In addition, the Gibbs free energy of the template compound (diphenyl-n-methylquinine 1) is very close to its possible degradation compound 4, and even lower than compounds 3 and 5. The Gibbs energy of compound 6 is lower than that of template compound 1, but the activation energy of the degradation reaction is very high (76.47 kCal/mol), which is much higher than the reported N, n-dimethyl assignment (15.23 kCal/mol). The above calculations show that n-methylquinine is more stable than N, n-dimethylpyridine (Figure 1c).
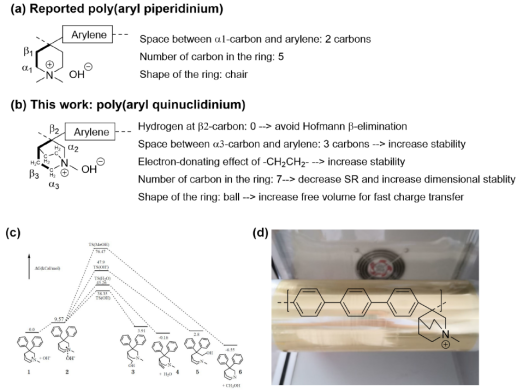
Figure 1. (a) The basic structure of the reported polyarylpyridine; (b) Structure of polyarylquinuclidinium in this study; (c) DFT calculation of the model compound diphenyl n-methylquinine under different degradation pathways; (d) Molecular structure of polytriphenylquinine and preparation of AEM.
 微信扫码 关注我们
微信扫码 关注我们

24小时咨询热线

移动电话13419663615
Copyright © 2022 All Rights Reserved. 地址:Rm 058,209,2F,Podium Bldg,C2 Bldg,Longshan Imno.Park,FutureSci.&Tech.City, No.999 GaoxinAve, Wuhan 430206 苏ICP123456 XML地图